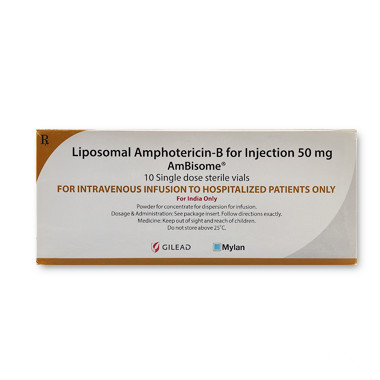
Ambisome(安必素)安必松仿制药是真的吗,安必素(Amphotericin)的版本有:1、美国吉利德版本;2、美国迈兰版本。代购价格是1600元左右,不同版本价格不同,以实际为准。请选择正规海外代购渠道,以保证产品质量。
Ambisome (安必素) is a generic version of the antifungal medication Amphotericin B. It is used for the treatment of various fungal infections. Now, let's take a closer look at whether Ambisome is genuine or not.
1. Introduction:
Amphotericin B is a widely used antifungal drug that has proven to be effective against severe fungal infections. Ambisome is a generic version of the brand name medication and is produced by various pharmaceutical companies. In this article, we will explore the authenticity of Ambisome and shed light on its use in treating fungal infections.
2. What is Ambisome?
Ambisome is a lipid-based formulation of Amphotericin B that is used to treat systemic fungal infections. It is administered intravenously and is predominantly prescribed for patients with compromised immune systems, such as those with HIV/AIDS or undergoing organ transplantation. The liposomal formulation of Ambisome reduces the toxicity associated with the conventional Amphotericin B, making it a preferred choice for certain patients.
3. Safety and Efficacy:
Generic medications are approved by regulatory authorities based on their equivalent safety and efficacy to the brand-name drug. In the case of Ambisome, the generic versions undergo rigorous testing to ensure they meet the required criteria. The regulatory authorities evaluate factors such as the drug's active ingredient, dosage form, bioequivalence, and manufacturing standards to determine its authenticity. Ambisome's generic versions are therefore expected to have similar safety and efficacy profiles as the brand-name drug.
4. Authorized Manufacturers:
To determine if a generic medication like Ambisome is genuine, it is crucial to identify the authorized manufacturers approved by the regulatory authorities. These manufacturers adhere to strict quality control measures and often have their facilities inspected and audited to ensure compliance with Good Manufacturing Practices (GMP). By verifying the manufacturer's credentials, patients can ensure that they are receiving an authentic version of Ambisome.
In conclusion, Ambisome (安必素) is indeed a genuine generic version of the antifungal medication Amphotericin B. It is approved by regulatory authorities based on its equivalent safety and efficacy to the brand-name drug. Patients can trust the authorized manufacturers who produce Ambisome to adhere to rigorous quality control measures. However, it is important to consult with healthcare professionals and obtain medication from reliable sources to ensure the authenticity and appropriate use of Ambisome in the treatment of fungal infections.